Volatile Organic Compounds (VOCs) are substances that form fine particulate matter (PM₂.₅) and ozone (O3) are key precursors that play a central role in atmospheric photochemical processes. There are various types of VOCs, mainly including alkanes, alkenes, halogenated hydrocarbons, aromatic hydrocarbons, and oxygen-containing organic compounds. They are widely sourced from petrochemical production, solvent use, traffic exhaust emissions, oil and gas storage and transportation, and industrial coating processes. Due to their high activity and complex chemical conversion pathways, VOCs not only promote the generation of photochemical smog, but also pose a serious threat to the ecological environment and human health.
Among them, although alkane compounds have relatively low chemical reactivity, they are abundant and emitted in the atmosphere. They can indirectly promote the formation of ozone and secondary organic aerosols (SOA) by reacting with hydroxyl radicals (· OH) to generate peroxide radicals. Long chain or branched alkanes can also generate secondary pollutants such as organic peroxides and aldehydes and ketones during the oxidation process, which have a sustained impact on air quality. Alkene VOCs have high unsaturation and much higher reactivity than alkanes, making them one of the main driving forces for atmospheric photochemical reactions. They are highly prone to react with · OH, ozone, or NO3 radicals under sunlight, generating photochemical smog, ozone, and various organic peroxides. Some low molecular weight olefins, such as ethylene and propylene, have high concentrations in urban atmosphere and contribute significantly to the Ozone Formation Potential (OFP); High carbon olefins or aromatic olefins may also generate aerosol particles through secondary reactions, increasing PM₂.₅ Load. Aromatic VOCs (such as benzene, toluene, xylene, ethylbenzene, etc.) not only have high toxicity and carcinogenicity, but also generate polycyclic aromatic hydrocarbons (PAHs) and secondary organic aerosols with strong adsorption and bioaccumulation during atmospheric oxidation. Benzene is classified as a Group I carcinogen by the World Health Organization (WHO), and long-term exposure can lead to hematopoietic system diseases and genetic mutations; Toluene and xylene can cause central nervous system damage, respiratory irritation, and endocrine disorders. Aromatic compounds also have strong light absorption ability and participate in the formation of atmospheric brown carbon, exacerbating regional haze pollution.
Overall, VOCs pose a dual threat to human health and atmospheric environment through direct toxic effects and complex photochemical conversion processes. Efficiently controlling VOCs emissions, optimizing source substitution processes, and strengthening end of pipe treatment technologies have become key directions for current air pollution prevention and fine management, as well as achieving regional ozone and PM2.5 The important foundation for collaborative emission reduction.
We have developed various advanced catalytic and control strategies to address the challenges of VOCs control, such as low efficiency, high energy consumption, and easy deactivation. By constructing unique structures such as single atoms, oxygen vacancies, and ordered macropores, a series of catalysts such as precious metal Pt based catalysts and transition metal Cu based catalysts have been developed. The catalytic active sites and mechanisms have been deeply explored, revealing the structure-activity relationship of the catalysts, and achieving efficient catalytic oxidation of VOCs at low or even room temperature, significantly reducing energy consumption. In addition, by innovatively utilizing the unique electronic structure of graphitic acetylene and sp hybridized carbon, composite materials such as MoO3/GDY and CuO/GDY have been successfully developed, enhancing the activity and stability of catalytic decomposition of ozone and oxidation of VOCs. Our work is dedicated to efficient and low-carbon control of volatile organic pollutants, providing clear guidance for the industrial development of efficient VOCs catalytic oxidation equipment.
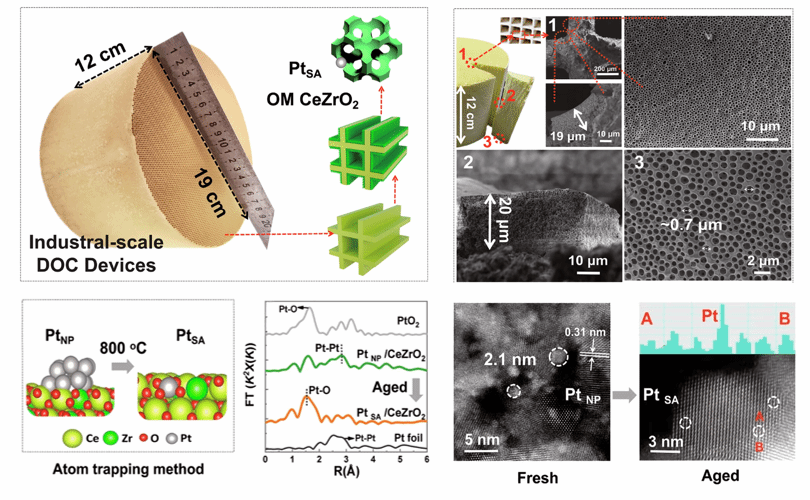
Figure (1) Preparation of high stability integral catalyst
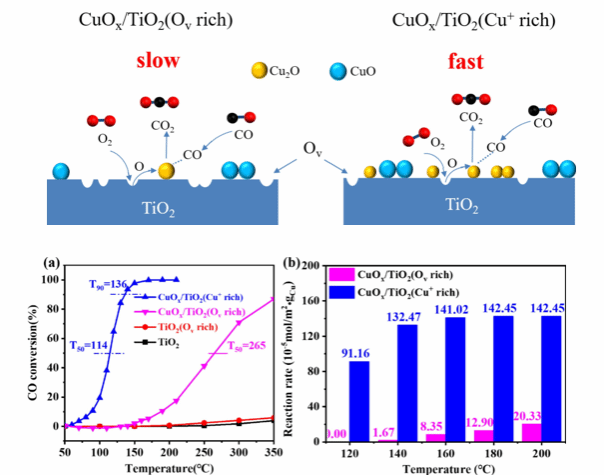
Figure (2) CuO/TiO₂ series catalysts
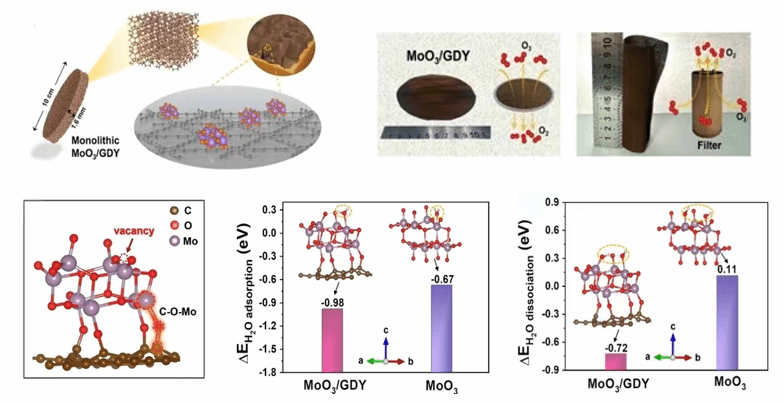
Figure (3) Metal Oxides/GDY composite material
Publish an article
◆ Nat Commun., 2025, 16, 7847-7860
◆ Environ. Sci. Technol., 2024, 58, 18020-18032
◆ J. Hazard. Mater., 2024, 480, 135849-135859
◆ Appl. Surf. Sci., 2023, 618, 156539-156548
◆ Angew. Chem. Int. Ed., 2023, 62, 9158-9166
◆ Environ. Sci. Technol., 2022,56, 3245-3257
◆ Nat. Commun., 2020, 11, 1062-1071
◆ Environ. Sci. Technol., 2020, 54, 15476-15488
◆ ACS Appl. Mater. Interfaces, 2020, 12, 7091-7101