In the fields of industrial manufacturing, energy supply, and agricultural production, the emissions of greenhouse gases such as C₃F₈、SF₆、CO₂、CH₄、N₂O, and N₂O are key factors driving global climate change and disrupting ecological balance. Among them, SF₆, C₃F₈ and other typical Electronic Specialty Gases are widely used in semiconductor manufacturing, display panel etching, power equipment insulation, and refrigeration systems. Although these gases play an irreplaceable role in industrial technology, their impact on the environment is extremely serious. Taking SF₆ as an example, its global warming potential (GWP) is about 23500 times that of CO₂, and its lifespan in the atmosphere can reach up to 3200 years; The GWP of C₃F₈ (perfluoropropane) is about 7000 times that of CO₂, and it also has strong greenhouse effect and extremely difficult decomposition characteristics. Once these fluorinated greenhouse gases escape into the atmosphere, they will accumulate for a long time and cause sustained damage to the Earth's radiation balance.
In contrast, although CO₂, CH₄, and N₂O are common carbon based greenhouse gases, they are also major drivers of global climate change due to their large emissions, wide sources, and high stability in the atmosphere. Carbon dioxide (CO₂) mainly comes from the combustion of fossil fuels, industrial processes, and deforestation, and is the core gas causing global temperature rise; The global warming potential of methane (CH4) is about 28 times that of CO₂, mainly derived from natural gas extraction, agriculture, animal husbandry, and landfills. It can also promote ozone generation in the troposphere and exacerbate air pollution; Nitrous oxide (N₂O) mainly comes from fertilizer use, soil microbial reactions, and industrial emissions. Its GWP is about 265 times that of CO₂, and it has a dual hazard of damaging the stratospheric ozone layer.
Overall, electronic gases (such as SF₆, C₃F₈) and carbon based greenhouse gases (such as CO₂, CH₄, N₂O) together constitute the main factors exacerbating the current greenhouse effect: the former is "highly efficient and powerful" but emits relatively small amounts, while the latter is "inefficient and massive" but in large quantities. The combined effect of two types of gases not only leads to a sustained increase in global average temperature, but also has profound impacts on ecosystem stability, agricultural production safety, and energy utilization efficiency. Therefore, strengthening greenhouse gas emissions reduction and promoting the research and recycling of low-carbon alternative gases have become key paths to achieving the "dual carbon" goals and addressing climate change.
Greenhouse gas capture and conversion technology can effectively control greenhouse gases and reduce their net emissions into the atmosphere. Therefore, our team is committed to developing new and efficient greenhouse gas control materials and processes based on existing research, improving capture efficiency and catalytic conversion performance, promoting the resource utilization of captured products, and achieving closed-loop operation of the technical path. This has significant strategic implications for addressing global climate change and advancing carbon neutrality goals.
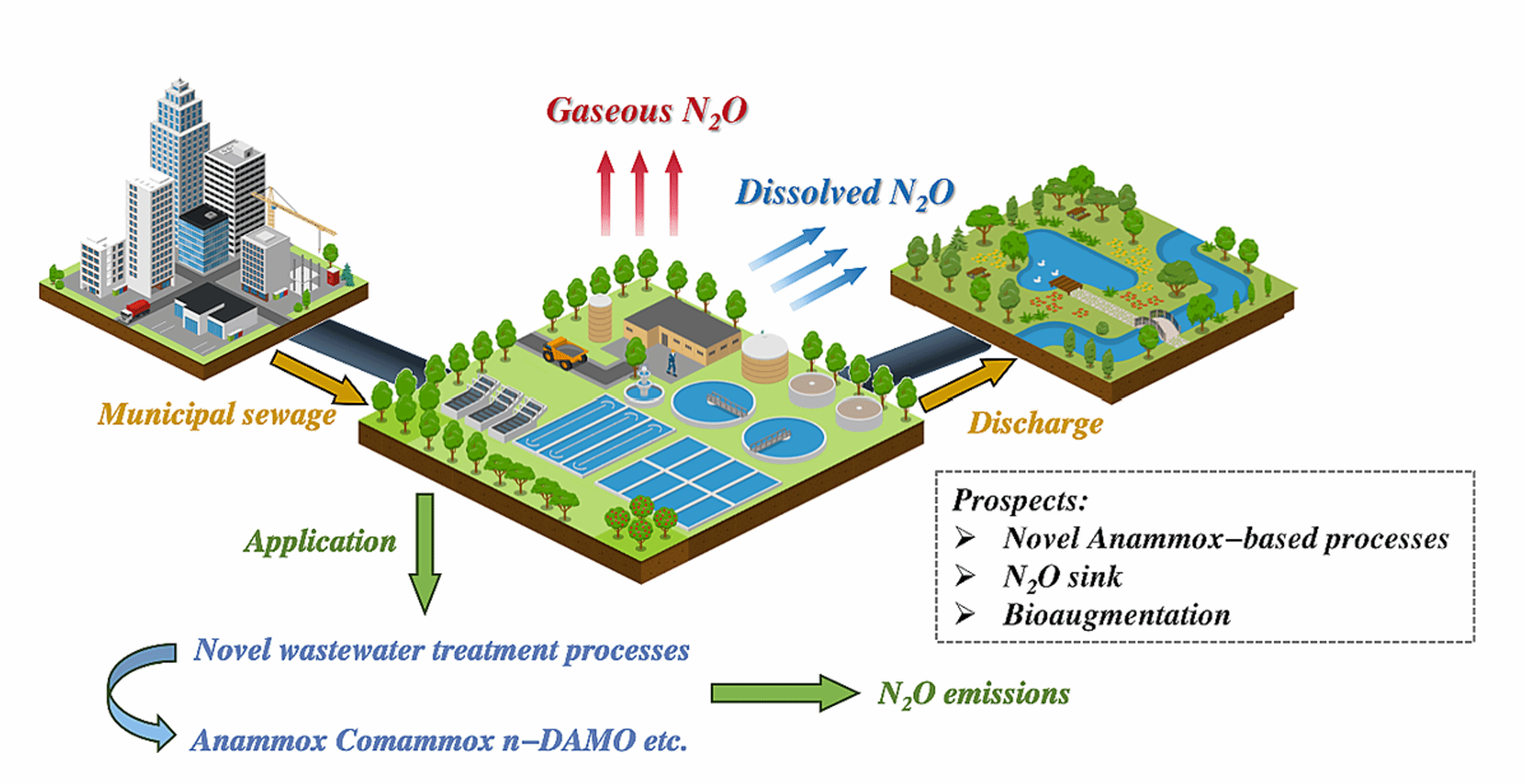
Figure (1) Path diagram of N2O generation
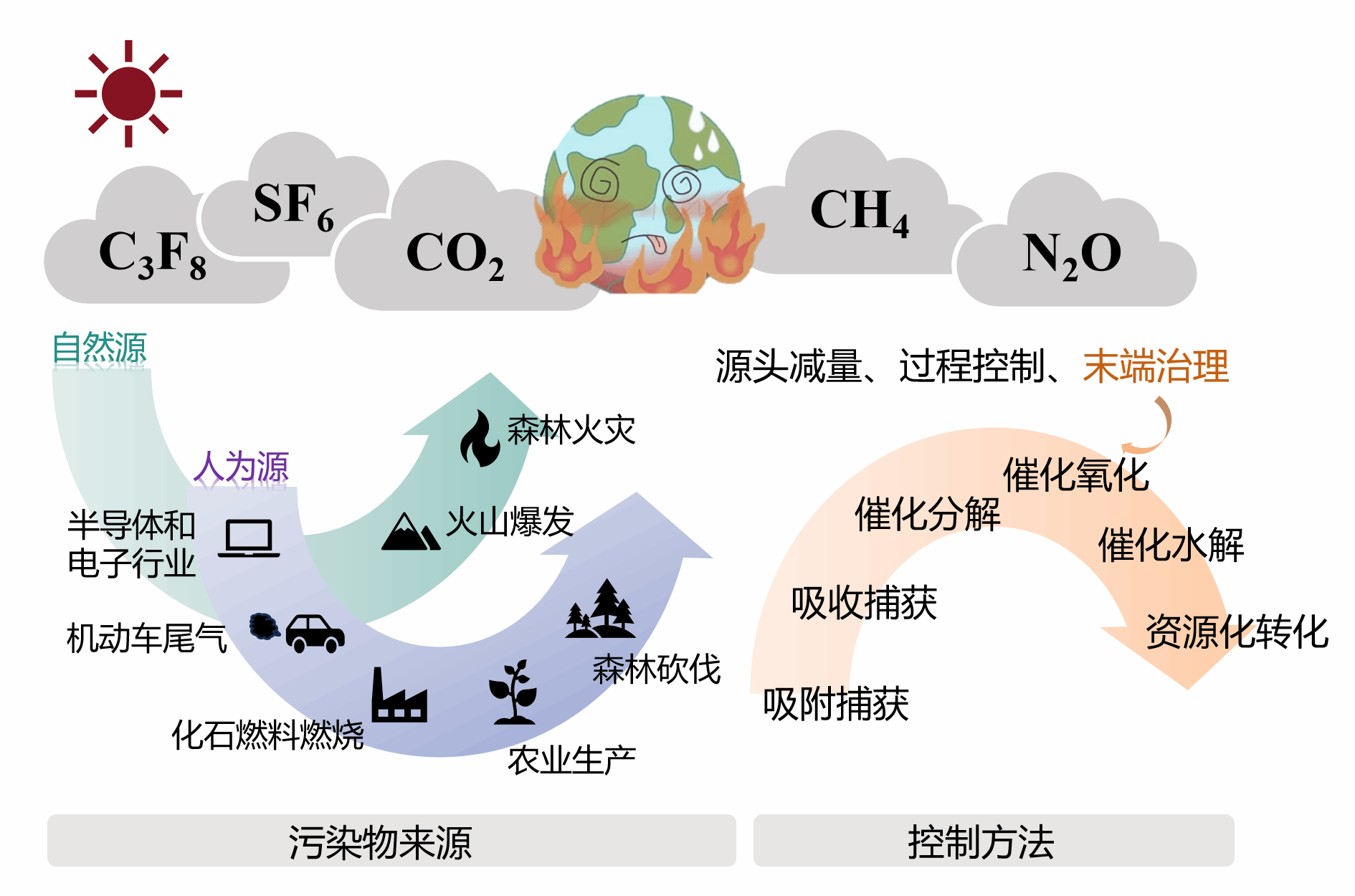
Figure (2) Sources of several common greenhouse gases
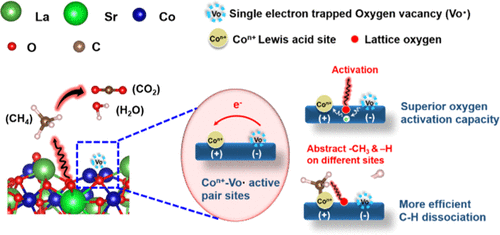
Figure (3) Perovskite based catalyst for catalytic oxidation of greenhouse gases
Publish an article
◆ Angew. Chem. Int. Ed. 2023, e202313868
◆ Chem. Eng. J., 2023, 451, 138930
◆ Environ. Sci. Technol., 2021, 55, 9243-9254
◆ Appl. Catal., B: Environ., 2020, 265, 118469
◆ Advanced Materials Interfaces, 2018, 5, 1700730-1700741
◆ Chin. Chem. Lett., 2018, 29, 252-260
◆ Angew. Chem. Int. Ed., 2014, 126, 7351-7355
Volatile Organic Compounds (VOCs) are substances that form fine particulate matter (PM₂.₅) and ozone (O3) are key precursors that play a central role in atmospheric photochemical processes. There are various types of VOCs, mainly including alkanes, alkenes, halogenated hydrocarbons, aromatic hydrocarbons, and oxygen-containing organic compounds. They are widely sourced from petrochemical production, solvent use, traffic exhaust emissions, oil and gas storage and transportation, and industrial coating processes. Due to their high activity and complex chemical conversion pathways, VOCs not only promote the generation of photochemical smog, but also pose a serious threat to the ecological environment and human health.
Among them, although alkane compounds have relatively low chemical reactivity, they are abundant and emitted in the atmosphere. They can indirectly promote the formation of ozone and secondary organic aerosols (SOA) by reacting with hydroxyl radicals (· OH) to generate peroxide radicals. Long chain or branched alkanes can also generate secondary pollutants such as organic peroxides and aldehydes and ketones during the oxidation process, which have a sustained impact on air quality. Alkene VOCs have high unsaturation and much higher reactivity than alkanes, making them one of the main driving forces for atmospheric photochemical reactions. They are highly prone to react with · OH, ozone, or NO3 radicals under sunlight, generating photochemical smog, ozone, and various organic peroxides. Some low molecular weight olefins, such as ethylene and propylene, have high concentrations in urban atmosphere and contribute significantly to the Ozone Formation Potential (OFP); High carbon olefins or aromatic olefins may also generate aerosol particles through secondary reactions, increasing PM₂.₅ Load. Aromatic VOCs (such as benzene, toluene, xylene, ethylbenzene, etc.) not only have high toxicity and carcinogenicity, but also generate polycyclic aromatic hydrocarbons (PAHs) and secondary organic aerosols with strong adsorption and bioaccumulation during atmospheric oxidation. Benzene is classified as a Group I carcinogen by the World Health Organization (WHO), and long-term exposure can lead to hematopoietic system diseases and genetic mutations; Toluene and xylene can cause central nervous system damage, respiratory irritation, and endocrine disorders. Aromatic compounds also have strong light absorption ability and participate in the formation of atmospheric brown carbon, exacerbating regional haze pollution.
Overall, VOCs pose a dual threat to human health and atmospheric environment through direct toxic effects and complex photochemical conversion processes. Efficiently controlling VOCs emissions, optimizing source substitution processes, and strengthening end of pipe treatment technologies have become key directions for current air pollution prevention and fine management, as well as achieving regional ozone and PM2.5 The important foundation for collaborative emission reduction.
We have developed various advanced catalytic and control strategies to address the challenges of VOCs control, such as low efficiency, high energy consumption, and easy deactivation. By constructing unique structures such as single atoms, oxygen vacancies, and ordered macropores, a series of catalysts such as precious metal Pt based catalysts and transition metal Cu based catalysts have been developed. The catalytic active sites and mechanisms have been deeply explored, revealing the structure-activity relationship of the catalysts, and achieving efficient catalytic oxidation of VOCs at low or even room temperature, significantly reducing energy consumption. In addition, by innovatively utilizing the unique electronic structure of graphitic acetylene and sp hybridized carbon, composite materials such as MoO3/GDY and CuO/GDY have been successfully developed, enhancing the activity and stability of catalytic decomposition of ozone and oxidation of VOCs. Our work is dedicated to efficient and low-carbon control of volatile organic pollutants, providing clear guidance for the industrial development of efficient VOCs catalytic oxidation equipment.
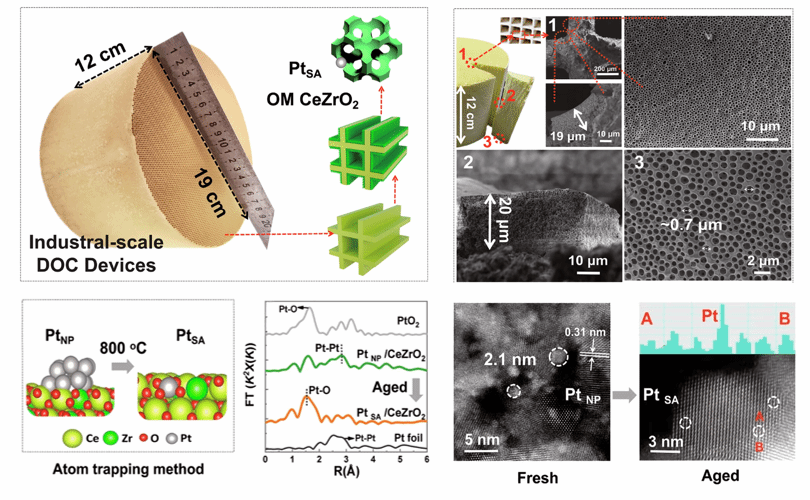
Figure (1) Preparation of high stability integral catalyst
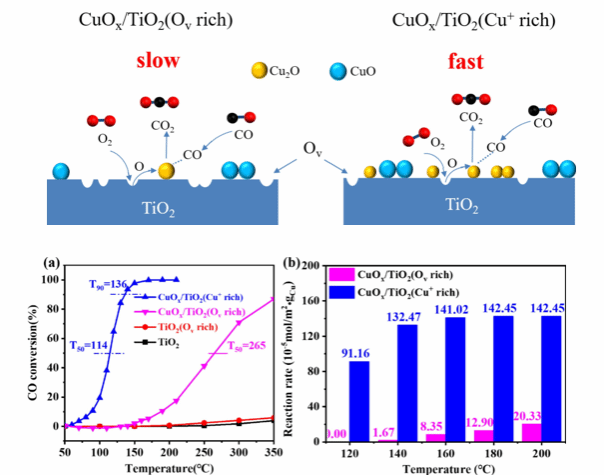
Figure (2) CuO/TiO₂ series catalysts
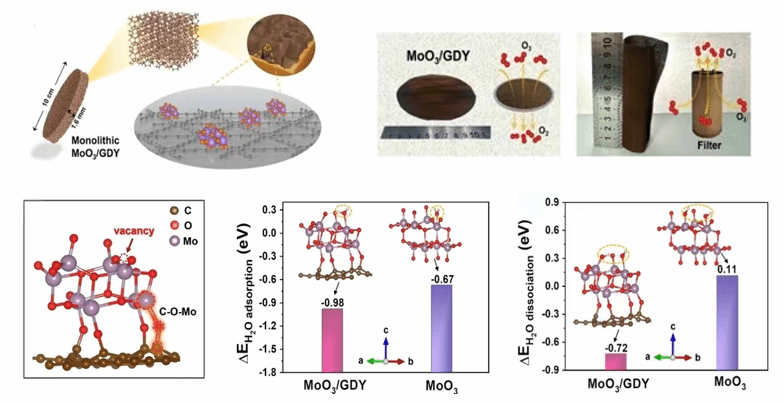
Figure (3) Metal Oxides/GDY composite material
Publish an article
◆ Nat Commun., 2025, 16, 7847-7860
◆ Environ. Sci. Technol., 2024, 58, 18020-18032
◆ J. Hazard. Mater., 2024, 480, 135849-135859
◆ Appl. Surf. Sci., 2023, 618, 156539-156548
◆ Angew. Chem. Int. Ed., 2023, 62, 9158-9166
◆ Environ. Sci. Technol., 2022,56, 3245-3257
◆ Nat. Commun., 2020, 11, 1062-1071
◆ Environ. Sci. Technol., 2020, 54, 15476-15488
◆ ACS Appl. Mater. Interfaces, 2020, 12, 7091-7101
Graphdiyne (GDY), as an emerging carbon allotrope, exhibits excellent properties distinct from traditional carbon materials such as graphene and carbon nanotubes due to its unique sp sp² hybrid conjugated structure. The lattice is connected by benzene ring units through - C ≡ C-alkyne bonds, forming a highly ordered two-dimensional π - conjugated network. This structure not only endows graphene acetylene with adjustable band structure and excellent electron transport performance, but also gives it a high specific surface area and uniformly distributed pore channels, providing ideal channels for the adsorption and migration of atoms, ions, and molecules.
It is worth noting that sp hybridized carbon atoms in graphitic alkynes have high unsaturation and strong chemical reactivity, which can generate stable coordination or electronic interactions with various metal atoms, effectively preventing metal aggregation and migration. This characteristic makes graphene acetylene an ideal carrier for constructing single atom catalysts (SACs). Its abundant alkyne bond sites not only provide high-density active centers for anchoring metal atoms, but also enable precise regulation of single atom electronic states and reaction pathways by adjusting the electronic structure of alkyne bonds, thereby significantly enhancing catalytic activity and selectivity. In addition, the regular pore structure and high specific surface area of graphitic acetylene contribute to achieving high loading single atom dispersion, while promoting efficient mass transfer of reactants and product desorption; Its excellent chemical stability and conductivity further ensure the long-term operational performance of the catalytic system under complex environmental conditions. Therefore, graphitic acetylene is not only a structurally innovative two-dimensional material, but also a new type of catalytic platform with designability and controllability.
Based on the significant advantages mentioned above, our team has been committed to the design and construction of new graphite alkyne based catalysts in recent years, systematically studying their intrinsic structure performance relationship, and exploring their potential applications in environmental catalysis, energy conversion, and pollution control. By regulating the electronic structure of graphene acetylene surface, constructing the metal carrier interface, and analyzing the reaction mechanism, we aim to reveal its unique catalytic mechanism and provide new theoretical basis and research ideas for the development of efficient and sustainable environmental catalysts.
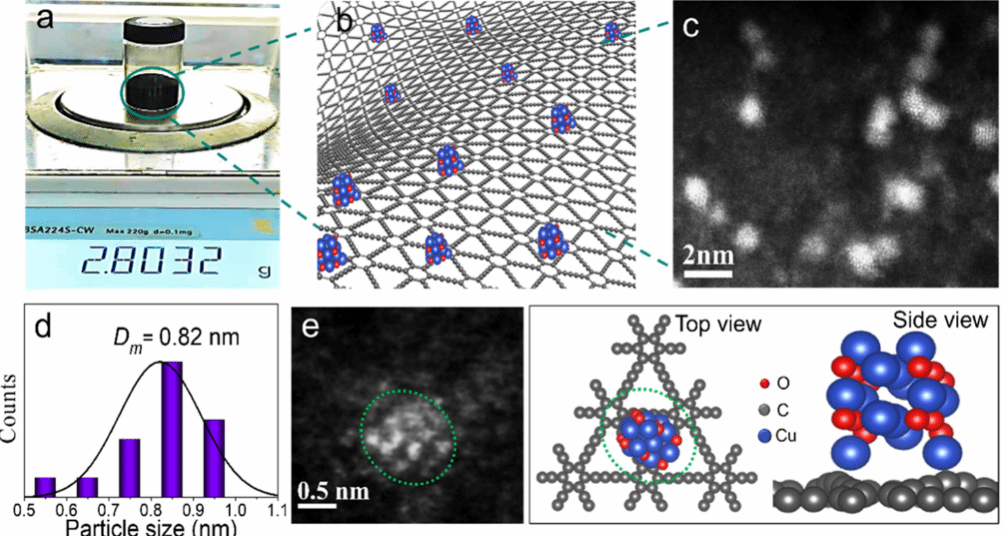
Figure (1) GDY based catalyst supported on CuO nanoclusters
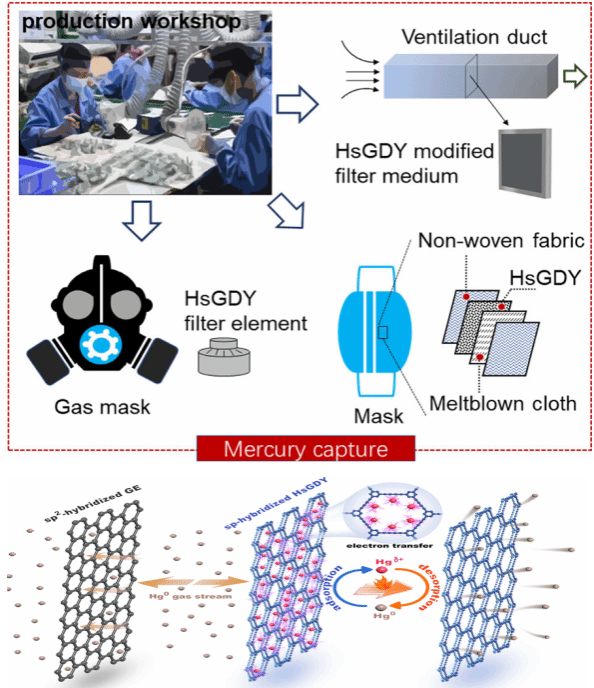
Figure (2) HsGDY material captures Hg0 in the air
Publish an article
◆ Chem. Sci., 2024, 15, 5061
◆ Nat. Commun. 2025, 16, 2439
◆ PNAS. 2023, 120(16), e2221002120
◆ Angew. Chem. Int. Ed., 2023, 62, e202309158
◆ J. Am. Chem. Soc., 2022, 144, 4942-4951
◆ J. Am. Chem. Soc., 2021, 143, 8720-8730
◆ Environ. Sci. Nano, 2021, 8, 1863-1885
◆ Molecules, 2020, 25, 18
◆ ACS Appl. Mater. Interfaces, 2018, 10, 17167-17174